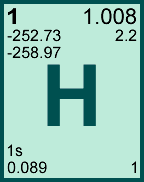
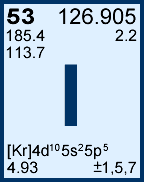
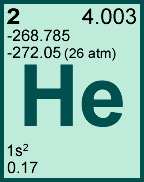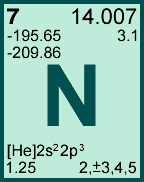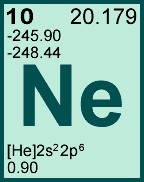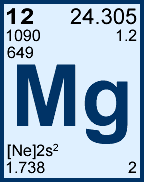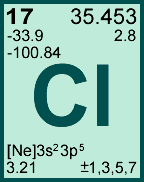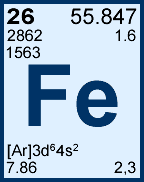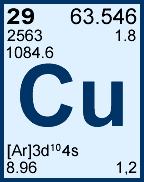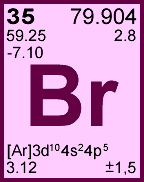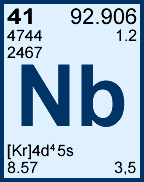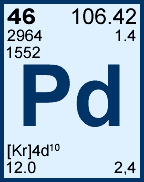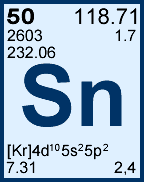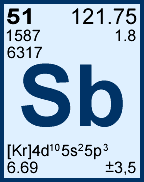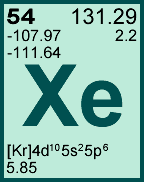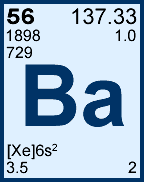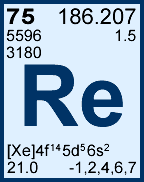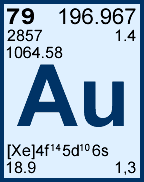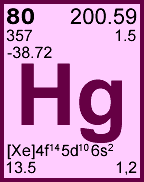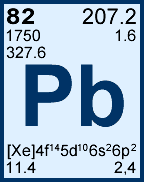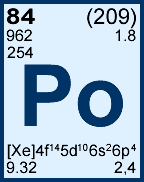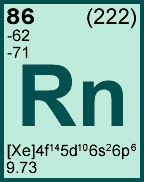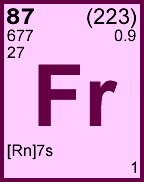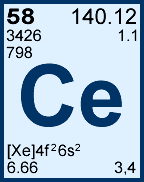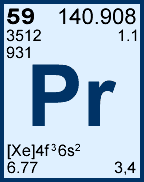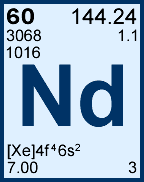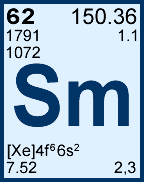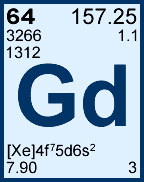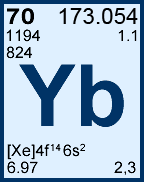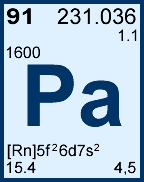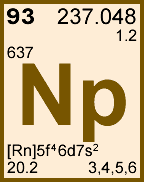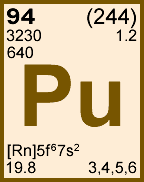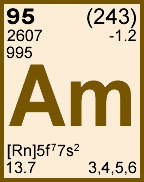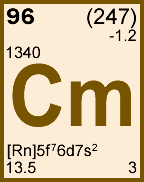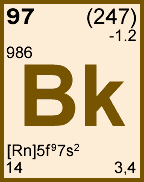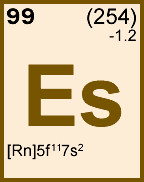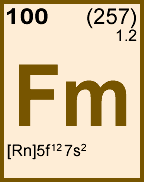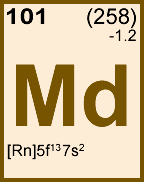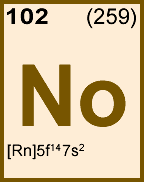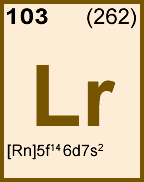Storage & Handling: Keep tightly sealed when not in use. Store and use at 20 ± 4°C. Do not pipet from container. Do not return portions removed for pipetting to container.
Chemical Compatibility: Soluble in HCl and HNO3. Avoid H2SO4 HF, H3PO4 and neutral to basic media. Stable with most metals and inorganic anions forming insoluble silicate, carbonate, hydroxide, oxide, fluoride, sulfate, oxalate, chromate, arsenate, and tungstate in neutral aqueous media.
Stability: 2-100 ppb levels stable for months in 1% HNO3 / LDPE container. 1-10,000 ppm solutions chemically stable for years in 1-10% HNO3 / LDPE container.
Ca Containing Samples (Preparation & Solution): Metal (best dissolved in diluted HNO3); Ores (carbonate fusion in Pt0 followed by HCl dissolution); Organic Matrices (dry ash and dissolution in dilute HCl. Do not heat when dissolving to avoid precipitation of SiO2). The oxide, hydroxide, carbonate, phosphate, and fluoride of calcium are soluble in % levels of HCl or HNO3. The sulfates (gypsum, anhydrite, etc.), certain silicates, and complex compounds require fusion with Na2CO3 followed by HCl / water dissolution. Note that contamination is a very real problem when analyzing for trace levels.